Khác biệt giữa bản sửa đổi của “Sinh khả dụng”
n Thêm thể loại, replaced: {{cite book → {{chú thích sách (4), {{reflist}} → {{tham khảo}} using AWB |
|||
| Dòng 30: | Dòng 30: | ||
== Tham khảo == |
== Tham khảo == |
||
{{ |
{{tham khảo}} |
||
== Tham khảo thêm== |
== Tham khảo thêm== |
||
* {{ |
* {{chú thích sách|author1=Malcolm Rowland|author2=Thomas N. Tozer|title=Clinical Pharmacokinetics and Pharmacodynamics: Concepts and Applications|edition=4|year=2010|publisher=Lippincott Williams & Wilkins|location=Philadelphia, PA|isbn=978-0-7817-5009-7}} |
||
* {{ |
* {{chú thích sách|author1=Peter G. Welling|author2=Francis L. S. Tse|author3=Shrikant V. Dighe|title=Pharmaceutical Bioequivalence|series=Drugs and the Pharmaceutical Sciences|volume=48|year=1991|publisher=Marcel Dekker|location=New York, NY|isbn=978-0-8247-8484-3}} |
||
* {{ |
* {{chú thích sách|author1=Dieter Hauschke|author2=Volker Steinijans|author3=Iris Pigeot|title=Bioequivalence Studies in Drug Development: Methods and Applications|url=http://books.google.com/books?id=mVOOyubcXqwC|accessdate=21 April 2011|series=Statistics in Practice|year=2007|publisher=John Wiley and Sons|location=Chichester, UK|isbn=978-0-470-09475-4|pages=17–36|chapter=Metrics to characterize concentration-time profiles in single- and multiple-dose bioequivalence studies|chapterurl=http://books.google.com/books?id=mVOOyubcXqwC&pg=PA17}} |
||
* {{ |
* {{chú thích sách|author1=Shein-Chung Chow|author2=Jen-pei Liu|title=Design and Analysis of Bioavailability and Bioequivalence Studies|edition=3|series=Biostatistics Series|volume=27|date=15 October 2008|publisher=CRC Press|location=Boca Raton, FL|isbn=978-1-58488-668-6}} |
||
== Liên kết ngoài == |
== Liên kết ngoài == |
||
Phiên bản lúc 18:44, ngày 24 tháng 3 năm 2013
Bài viết này là một bài mồ côi vì không có bài viết khác liên kết đến nó. Vui lòng tạo liên kết đến bài này từ các bài viết liên quan; có thể thử dùng công cụ tìm liên kết. (tháng 3 2013) |
Trong Dược học, Sinh khả dụng là một địa lượng chỉ tốc độ và mức độ hấp thu dược chất từ một chế phầm bào chế vào tuần hoàn chung một cách nguyên vẹn và đưa đến nơi tác dụng.[1]
Được ký hiệu bằng chữ f (hay nếu ở dạng phần trăm là F).
Định nghĩa
Trong Dược học, Sinh khả dụng (hay như trong tiếng Anh là bioavailability (BA)) là đại lượng chỉ tốc độ và mức độ hấp thu dược chất từ một chế phẩm bào chế và tuần hoàn chung một cách nguyên vẹn và đưa đến nơi tác dụng, từ đó tiếp tục được chuyển hoá và thải hồi. Từ định nghĩa, thuốc theo đường tiêm tĩnh mạch có sinh khả dụng là 100%.[2] Tuy vậy, khi thuốc được dùng bằng các cách thức khác nhau (như đường uống) thì sinh khả dụng của thuốc[TH] thường giảm (do hấp thu không hoàn toàn và các giai đoạn đầu của quá trình trao đỗi chất) hay thay đỗi tuỳ thuộc thể trạng bệnh nhân. Sinh khả dụng được xem là một công cụ thiết yếu trong sinh dược học, đây là đại lượng quan trọng để xác định và tính toán liều dùng cho các dạng bào chế không theo đường tĩnh mạch.
Sinh khả dụng tuyệt đối
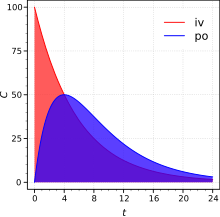
Sinh khả dụng tuyệt đối được xác định khi so sánh sinh khả dụng của thuốc có hoạt tính lưu hành trong hệ tuần hoàn theo cong đường không phải tiêm tĩnh mạch (ví dụ như theo đường uống, đường trực tràng, thẩm thấu qua da, tiêm dưới da hay đạt dưới lưỡi), với sinh khả dụng của cùng dạng thuốc theo đường tiêm tĩnh mạch. Do chỉ có một phần của thuốc hấp thụ theo đường không tiêm tĩnh mạch so với củng dạng thuốc đó khi tiêm tĩnh mạch, nên sự so sánh này phải được thực hiện trên cách liều khác nhau (ví dụ, khảo sát các liều dùng khác nhau hay trên các đối tượng có trọng lượng khác nhau). Từ đó , nồng độ chất hấp thụ sẻ dần được nâng cao bằng cách chia liệu lượng dùng hợp lý.
Để xác định được sinh khả dụng tuyệt đối của một loại thuốc ta cần xác định được mối liên hệ giữa thời gian và nồng độ của thuốc lưu hành trong huyết tuơng. Để biết được mối tương quan trên trước hết phải xác định được liều dùng theo đường tiêm tĩnh mạch (IV) và cả đường không tiêm tĩnh mạch (ví dụ: đường uống) administration. Sinh khả dụng là liều dùng xác định thể hiện bằng diện tích dưới đường cong (AUC) theo đường không tiêm tĩnh mạch chia cho diện tích dưới đường cong AUC theo đường tiêm tĩnh mạch. Ví dụ, công thức tính F (sinh khả dụng) của một loại thuốc được dùng theo đường uống (po) được cho như sau.
Ghi chú
Xem thêm
Tham khảo
- ^ Shargel, L.; Yu, A.B. (1999). Applied biopharmaceutics & pharmacokinetics (4th ed.). New York: McGraw-Hill. ISBN 0-8385-0278-4Bản mẫu:Pn
- ^ Griffin, J.P. The Textbook of Pharmaceutical Medicine (6th Ed.). New Jersey: BMJ Books. ISBN 978-1-4051-8035-1Bản mẫu:Pn
- ^ Schuppan, D; Molz, KH; Staib, AH; Rietbrock, N (1981). “Bioavailability of theophylline from a sustained-release aminophylline formulation (Euphyllin retard tablets)--plasma levels after single and multiple oral doses”. International journal of clinical pharmacology, therapy, and toxicology. 19 (5): 223–7. PMID 7251238.
Tham khảo thêm
- Malcolm Rowland; Thomas N. Tozer (2010). Clinical Pharmacokinetics and Pharmacodynamics: Concepts and Applications (ấn bản 4). Philadelphia, PA: Lippincott Williams & Wilkins. ISBN 978-0-7817-5009-7.
- Peter G. Welling; Francis L. S. Tse; Shrikant V. Dighe (1991). Pharmaceutical Bioequivalence. Drugs and the Pharmaceutical Sciences. 48. New York, NY: Marcel Dekker. ISBN 978-0-8247-8484-3.
- Dieter Hauschke; Volker Steinijans; Iris Pigeot (2007). “Metrics to characterize concentration-time profiles in single- and multiple-dose bioequivalence studies”. Bioequivalence Studies in Drug Development: Methods and Applications. Statistics in Practice. Chichester, UK: John Wiley and Sons. tr. 17–36. ISBN 978-0-470-09475-4. Truy cập ngày 21 tháng 4 năm 2011. Đã bỏ qua tham số không rõ
|chapterurl=(trợ giúp) - Shein-Chung Chow; Jen-pei Liu (15 tháng 10 năm 2008). Design and Analysis of Bioavailability and Bioequivalence Studies. Biostatistics Series. 27 (ấn bản 3). Boca Raton, FL: CRC Press. ISBN 978-1-58488-668-6.

