Thành viên:Naazulene/Hợp chất thơm
Bài viết này đang bị thiếu thông tin về Non-benzenoid aromatc compounds. (October 2022) |
Hợp chất thơm hay arene là những hydrocarbon chứa một hoặc nhiều vòng thơm.[1] Gọi là "thơm" là vì người xưa hay phân loại chất theo mùi hương, trước khi những tính chất hóa học vẫn chưa được nghiên cứu kĩ. Định nghĩa hiện tại của hợp chất thơm không liên quan gì đến mùi hương của nó nữa.
Heteroarene là một nhóm chất liên quan, trong đó một phân tử carbon của arene bị thay thể bởi một dị nguyên tử (nguyên tử không phải carbon hay hydrogen, như oxygen, nitrogen hay lưu huỳnh). Mốt số heteroarene có tính chất của hợp chất thơm là furan và pyridine.
Hydrocarbon không có vòng là hợp chất không vòng (aliphatic). Cho đến năm 2000, khoảng một nửa hợp chất đã được phát hiện là hợp chất thơm.[2]
Hợp chất thơm có vai trò quan trọng trong cấu tạo hóa sinh của tất cả dạng sống, vì nó bao gồm:
- Amino acid thơm: có 4 amino acid thơm tham gia cấu tạo protein (cùng với 16 amino acid khác) là histidine, phenylalanine, tryptophan, và tyrosine.
- Nucleotide: cả 5 nucleotide tham gia cấu tạo DNA và RNA (adenine, thymine, cytosine, guanine, and uracil) đều là chứa vòng thơm.
- Heme: phân tử heme chưa hệ thống thơm với 22 π-electron.
- Diệp lục: chất diệp lục cũng có một hệ thống thơm tương tự heme.
Hợp chất thơm cũng rất quan trọng trong công nghiệp. Những hợp chất thơm có tính thương mại cao là benzene, toluene, ortho-xylene và para-xylene. Chúng được phân tách từ những hỗn hợp phức tạp qua quá trình tinh chế dầu hoặc chưng cất hắc ín, và được sử dụng để sản xuất một loạt chất hóa học quan trọng, bao gồm styrene, phenol, aniline, polyester và nylon.
Benzene ring model[sửa | sửa mã nguồn]
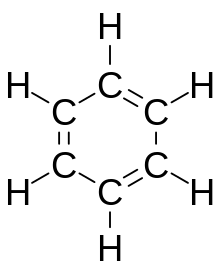
Benzene, , is the least complex aromatic hydrocarbon, and it was the first one named as such. The nature of its bonding was first recognized by August Kekulé in the 19th century. Each carbon atom in the hexagonal cycle has four electrons to share. One goes to the hydrogen atom, and one to each of the two neighboring carbons. This leaves one electron to share with one of the two neighboring carbon atoms, thus creating a double bond with one carbon and leaving a single bond with the other, which is why some representations of the benzene molecule portray it as a hexagon with alternating single and double bonds.
Other depictions of the structure portray the hexagon with a circle inside it, to indicate that the six electrons are floating around in delocalized molecular orbitals the size of the ring itself. This represents the equivalent nature of the six carbon-carbon bonds all of bond order 1.5; the equivalency is explained by resonance forms. The electrons are visualized as floating above and below the ring, with the electromagnetic fields they generate acting to keep the ring flat.
General properties of aromatic hydrocarbons:
- They display aromaticity
- The carbon-hydrogen ratio is high
- They burn with a strong sooty yellow flame because of the high carbon–hydrogen ratio
- They undergo electrophilic substitution reactions and nucleophilic aromatic substitutions
The circle symbol for aromaticity was introduced by Sir Robert Robinson and his student James Armit in 1925[3] and popularized starting in 1959 by the Morrison & Boyd textbook on organic chemistry. The proper use of the symbol is debated: some publications use it to any cyclic π system, while others use it only for those π systems that obey Hückel's rule. Jensen[4] argues that, in line with Robinson's original proposal, the use of the circle symbol should be limited to monocyclic 6 π-electron systems. In this way the circle symbol for a six-center six-electron bond can be compared to the Y symbol for a three-center two-electron bond.
Reactions[sửa | sửa mã nguồn]
Aromatic ring systems participate in many organic reactions.
Aromatic substitution[sửa | sửa mã nguồn]
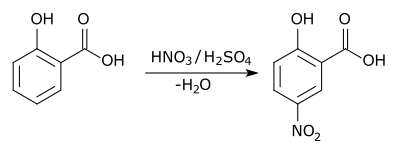
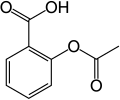
In aromatic substitution one substituent on the arene ring, usually hydrogen, is replaced by another substituent. The two main types are electrophilic aromatic substitution, when the active reagent is an electrophile, and nucleophilic aromatic substitution, when the reagent is a nucleophile. In radical-nucleophilic aromatic substitution, the active reagent is a radical. An example of electrophilic aromatic substitution is the nitration of salicylic acid:[5]
Coupling reactions[sửa | sửa mã nguồn]
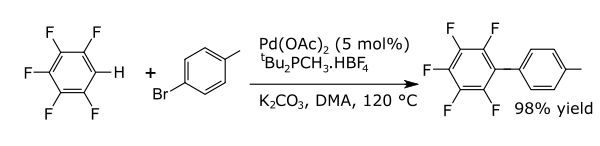
In coupling reactions a metal catalyzes a coupling between two formal radical fragments. Common coupling reactions with arenes result in the formation of new carbon–carbon bonds e.g., alkylarenes, vinyl arenes, biraryls, new carbon–nitrogen bonds (anilines) or new carbon–oxygen bonds (aryloxy compounds). An example is a direct arylation of perfluorobenzenes[6]
Hydrogenation[sửa | sửa mã nguồn]
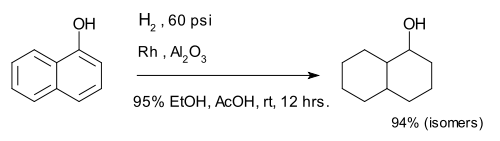
Hydrogenation of arenes create saturated rings. The compound 1-naphthol is completely reduced to a mixture of decalin-ol isomers.[7]
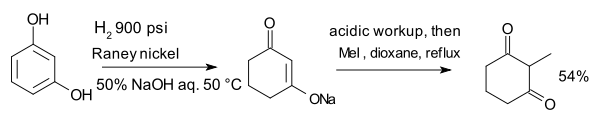
The compound resorcinol, hydrogenated with Raney nickel in presence of aqueous sodium hydroxide forms an enolate which is alkylated with methyl iodide to 2-methyl-1,3-cyclohexandione:[8]
Cycloadditions[sửa | sửa mã nguồn]
Cycloaddition reactions are not common. Unusual thermal Diels–Alder reactivity of arenes can be found in the Wagner-Jauregg reaction. Other photochemical cycloaddition reactions with alkenes occur through excimers.
Dearomatization[sửa | sửa mã nguồn]
In dearomatization reactions the aromaticity of the reactant is permanently lost.
Benzene and derivatives of benzene[sửa | sửa mã nguồn]
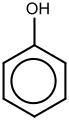
Benzene derivatives have from one to six substituents attached to the central benzene core. Examples of benzene compounds with just one substituent are phenol, which carries a hydroxyl group, and toluene with a methyl group. When there is more than one substituent present on the ring, their spatial relationship becomes important for which the arene substitution patterns ortho, meta, and para are devised. For example, three isomers exist for cresol because the methyl group and the hydroxyl group can be placed next to each other (ortho), one position removed from each other (meta), or two positions removed from each other (para). Xylenol has two methyl groups in addition to the hydroxyl group, and, for this structure, 6 isomers exist.
- Representative arene compounds
The arene ring has the ability to stabilize charges. This is seen in, for example, phenol (C6H5–OH), which is acidic at the hydroxyl (OH), since a charge on this oxygen (alkoxide –O−) is partially delocalized into the benzene ring.
Other monocyclic aromatic hydrocarbon[sửa | sửa mã nguồn]
Other monocyclic aromatic hydrocarbon include Cyclotetradecaheptaene or Cyclooctadecanonaene.
Polycyclic aromatic hydrocarbons[sửa | sửa mã nguồn]
Polynuclear aromatic hydrocarbons (PAHs) are aromatic hydrocarbons that consist of fused aromatic rings and do not contain heteroatoms or carry substituents.[9] Naphthalene is the simplest example of a PAH. PAHs occur in oil, coal, and tar deposits, and are produced as byproducts of fuel burning (whether fossil fuel or biomass). As pollutants, they are of concern because some compounds have been identified as carcinogenic, mutagenic, and teratogenic. PAHs are also found in cooked foods. Studies have shown that high levels of PAHs are found, for example, in meat cooked at high temperatures such as grilling or barbecuing, and in smoked fish.[10][11][12]
They are also found in the interstellar medium, in comets, and in meteorites and are a candidate molecule to act as a basis for the earliest forms of life. In graphene the PAH motif is extended to large 2D sheets.
See also[sửa | sửa mã nguồn]
- Aromatic substituents: Aryl, Aryloxy and Arenediyl
- Asphaltene
- Hydrodealkylation
- Simple aromatic rings
- Rhodium-platinum oxide, a catalyst used to hydrogenate aromatic compounds.
References[sửa | sửa mã nguồn]
- ^ “The IUPAC Compendium of Chemical Terminology”.
- ^ Balaban, Alexandru T.; Oniciu, Daniela C.; Katritzky, Alan R. (2004). “Aromaticity as a Cornerstone of Heterocyclic Chemistry”. Chemical Reviews. 104 (5): 2777–2812. doi:10.1021/cr0306790. PMID 15137807.
- ^ Armit, James Wilkins; Robinson, Robert (1925). “Polynuclear heterocyclic aromatic types. Part II. Some anhydronium bases”. J. Chem. Soc. Trans. 127: 1604–1618. doi:10.1039/CT9252701604.
- ^ Jensen, William B. (tháng 4 năm 2009). “The circle symbol for aromaticity” (PDF). J. Chem. Educ. 86 (4): 423–424. Bibcode:2009JChEd..86..423J. doi:10.1021/ed086p423. Lưu trữ (PDF) bản gốc ngày 9 tháng 10 năm 2022.
- ^ Webb, K.; Seneviratne, V. (1995). “A mild oxidation of aromatic amines”. Tetrahedron Letters. 36 (14): 2377–2378. doi:10.1016/0040-4039(95)00281-G. S2CID 93522679.
- ^ Lafrance, M.; Rowley, C.; Woo, T.; Fagnou, K. (2006). “Catalytic intermolecular direct arylation of perfluorobenzenes”. Journal of the American Chemical Society. 128 (27): 8754–8756. CiteSeerX 10.1.1.631.607. doi:10.1021/ja062509l. PMID 16819868.
- ^ Meyers, A. I.; Beverung, W. N.; Gault, R. “1-Naphthol”. Organic Syntheses. 51: 103.; Collective Volume, 6
- ^ Noland, Wayland E.; Baude, Frederic J. “Ethyl Indole-2-carboxylate”. Organic Syntheses. 41: 56.; Collective Volume, 5
- ^ Fetzer, J. C. (2000). “The Chemistry and Analysis of the Large Polycyclic Aromatic Hydrocarbons”. Polycyclic Aromatic Compounds. New York: Wiley. 27 (2): 143. doi:10.1080/10406630701268255. ISBN 0-471-36354-5. S2CID 97930473.
- ^ “Polycyclic Aromatic Hydrocarbons – Occurrence in foods, dietary exposure and health effects” (PDF). European Commission, Scientific Committee on Food. 4 tháng 12 năm 2002. Lưu trữ (PDF) bản gốc ngày 9 tháng 10 năm 2022.
- ^ Larsson, B. K.; Sahlberg, GP; Eriksson, AT; Busk, LA (1983). “Polycyclic aromatic hydrocarbons in grilled food”. J. Agric. Food Chem. 31 (4): 867–873. doi:10.1021/jf00118a049. PMID 6352775.
- ^ “Polycyclic Aromatic Hydrocarbons (PAHs)”. Agency for Toxic Substances and Disease Registry. 1996.
External links[sửa | sửa mã nguồn]
Bản mẫu:Hydrocarbons Bản mẫu:Aryl hydrocarbon receptor modulators Bản mẫu:Hallucinogens