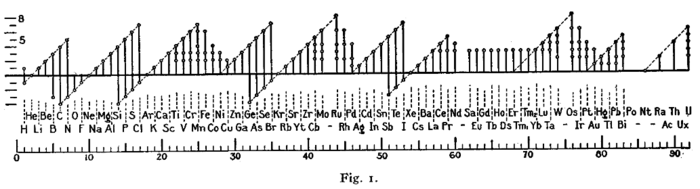
Danh sách trạng thái oxy hóa của các nguyên tố
Đây là danh sách các trạng thái oxy hóa được biết đến của các nguyên tố hóa học, ngoại trừ các giá trị không nguyên. Các trạng thái phổ biến nhất được in đậm. Bảng này dựa trên cơ sở của Greenwood và Earnshaw,[1] với các bổ sung ghi nhận. Trạng thái oxy hóa 0, xảy ra ở tất cả các nguyên tố, được ngụ ý bởi cột có biểu tượng của các nguyên tố đó. Định dạng của bảng do Dmitri Mendeleev đưa ra năm 1889 cho thấy sự tuần hoàn của các trạng thái oxy hóa của các nguyên tố.[1]
Danh sách
[sửa | sửa mã nguồn]Đậm: Trạng thái oxy hóa phổ biến
| Z | Nguyên tố | Trạng thái oxy hóa âm |
Trạng thái oxy hóa dương |
Nhóm | Ghi chú | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −5 | −4 | −3 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | +7 | +8 | +9 | ||||
| 1 | Hydro | −1 | H | +1 | 1 | |||||||||||||
| 2 | Heli | He | 18 | |||||||||||||||
| 3 | Lithi | Li | +1 | 1 | [2] | |||||||||||||
| 4 | Beryli | Be | +1 | +2 | 2 | [3] | ||||||||||||
| 5 | Bor | −5 | −1 | B | +1 | +2 | +3 | 13 | [4][5] | |||||||||
| 6 | Carbon | −4 | −3 | −2 | −1 | C | +1 | +2 | +3 | +4 | 14 | |||||||
| 7 | Nitơ | −3 | −2 | −1 | N | +1 | +2 | +3 | +4 | +5 | 15 | |||||||
| 8 | Oxy | −2 | −1 | O | +1 | +2 | 16 | |||||||||||
| 9 | Fluor | −1 | F | 17 | ||||||||||||||
| 10 | Neon | Ne | 18 | |||||||||||||||
| 11 | Natri | −1 | Na | +1 | 1 | [2] | ||||||||||||
| 12 | Magiê | Mg | +1 | +2 | 2 | [6] | ||||||||||||
| 13 | Nhôm | −2 | −1 | Al | +1 | +2 | +3 | 13 | [7][8][9] | |||||||||
| 14 | Silic | −4 | −3 | −2 | −1 | Si | +1 | +2 | +3 | +4 | 14 | |||||||
| 15 | Phosphor | −3 | −2 | −1 | P | +1 | +2 | +3 | +4 | +5 | 15 | |||||||
| 16 | Lưu huỳnh | −2 | −1 | S | +1 | +2 | +3 | +4 | +5 | +6 | 16 | |||||||
| 17 | Chlor | −1 | Cl | +1 | +2 | +3 | +4 | +5 | +6 | +7 | 17 | [10] | ||||||
| 18 | Argon | Ar | 18 | |||||||||||||||
| 19 | Kali | −1 | K | +1 | 1 | [2] | ||||||||||||
| 20 | Calci | Ca | +1 | +2 | 2 | [11] | ||||||||||||
| 21 | Scandi | Sc | +1 | +2 | +3 | 3 | [12][13] | |||||||||||
| 22 | Titan | −2 | −1 | Ti | +1 | +2 | +3 | +4 | 4 | [14][15][16] | ||||||||
| 23 | Vanadi | −3 | −1 | V | +1 | +2 | +3 | +4 | +5 | 5 | [15] | |||||||
| 24 | Crom | −4 | −2 | −1 | Cr | +1 | +2 | +3 | +4 | +5 | +6 | 6 | [15] | |||||
| 25 | Mangan | −3 | −2 | −1 | Mn | +1 | +2 | +3 | +4 | +5 | +6 | +7 | 7 | |||||
| 26 | Sắt | −4 | −2 | −1 | Fe | +1 | +2 | +3 | +4 | +5 | +6 | +7 | +8 | 8 | [17][18][19] | |||
| 27 | Cobalt | −3 | −1 | Co | +1 | +2 | +3 | +4 | +5 | 9 | [15] | |||||||
| 28 | Nickel | −2 | −1 | Ni | +1 | +2 | +3 | +4 | 10 | [20] | ||||||||
| 29 | Đồng | −2 | Cu | +1 | +2 | +3 | +4 | 11 | [19] | |||||||||
| 30 | Kẽm | −2 | Zn | +1 | +2 | 12 | [19][21] | |||||||||||
| 31 | Gali | −5 | −4 | −2 | −1 | Ga | +1 | +2 | +3 | 13 | [8][22] | |||||||
| 32 | Germani | −4 | −3 | −2 | −1 | Ge | +1 | +2 | +3 | +4 | 14 | [23] | ||||||
| 33 | Arsen | −3 | −2 | −1 | As | +1 | +2 | +3 | +4 | +5 | 15 | [8][24][25] | ||||||
| 34 | Seleni | −2 | −1 | Se | +1 | +2 | +3 | +4 | +5 | +6 | 16 | [26][27][28][29] | ||||||
| 35 | Brom | −1 | Br | +1 | +3 | +4 | +5 | +7 | 17 | |||||||||
| 36 | Krypton | Kr | +2 | 18 | ||||||||||||||
| 37 | Rubidi | −1 | Rb | +1 | 1 | [2] | ||||||||||||
| 38 | Stronti | Sr | +1 | +2 | 2 | [30] | ||||||||||||
| 39 | Ytri | Y | +1 | +2 | +3 | 3 | [31][32] | |||||||||||
| 40 | Zirconi | −2 | Zr | +1 | +2 | +3 | +4 | 4 | [15][33] | |||||||||
| 41 | Niobi | −3 | −1 | Nb | +1 | +2 | +3 | +4 | +5 | 5 | [15][34] | |||||||
| 42 | Molypden | −4 | −2 | −1 | Mo | +1 | +2 | +3 | +4 | +5 | +6 | 6 | [15] | |||||
| 43 | Techneti | −3 | −1 | Tc | +1 | +2 | +3 | +4 | +5 | +6 | +7 | 7 | ||||||
| 44 | Rutheni | −4 | −2 | Ru | +1 | +2 | +3 | +4 | +5 | +6 | +7 | +8 | 8 | [15][19] | ||||
| 45 | Rhodi | −3 | −1 | Rh | +1 | +2 | +3 | +4 | +5 | +6 | 9 | [15] | ||||||
| 46 | Paladi | Pd | +1 | +2 | +3 | +4 | 10 | [35][36] | ||||||||||
| 47 | Bạc | −2 | −1 | Ag | +1 | +2 | +3 | 11 | [19][37] | |||||||||
| 48 | Cadmi | −2 | Cd | +1 | +2 | 12 | [19][38] | |||||||||||
| 49 | Indi | −5 | −2 | −1 | In | +1 | +2 | +3 | 13 | [8][39][40] | ||||||||
| 50 | Thiếc | −4 | −3 | −2 | −1 | Sn | +1 | +2 | +3 | +4 | 14 | [8][41][42] | ||||||
| 51 | Antimon | −3 | −2 | −1 | Sb | +1 | +2 | +3 | +4 | +5 | 15 | [8][43][44][45] | ||||||
| 52 | Teluri | −2 | −1 | Te | +1 | +2 | +3 | +4 | +5 | +6 | 16 | [8][46][47][48] | ||||||
| 53 | Iod | −1 | I | +1 | +3 | +4 | +5 | +6 | +7 | 17 | [49][50] | |||||||
| 54 | Xenon | Xe | +2 | +4 | +6 | +8 | 18 | [51] | ||||||||||
| 55 | Caesi | −1 | Cs | +1 | 1 | [2] | ||||||||||||
| 56 | Bari | Ba | +1 | +2 | 2 | [52] | ||||||||||||
| 57 | Lanthan | La | +1 | +2 | +3 | 3 | [53] | |||||||||||
| 58 | Xeri | Ce | +2 | +3 | +4 | |||||||||||||
| 59 | Praseodymi | Pr | +2 | +3 | +4 | +5 | [54] | |||||||||||
| 60 | Neodymi | Nd | +2 | +3 | +4 | [55] | ||||||||||||
| 61 | Promethi | Pm | +2 | +3 | [56] | |||||||||||||
| 62 | Samari | Sm | +2 | +3 | ||||||||||||||
| 63 | Europi | Eu | +2 | +3 | ||||||||||||||
| 64 | Gadolini | Gd | +1 | +2 | +3 | |||||||||||||
| 65 | Terbi | Tb | +1 | +2 | +3 | +4 | [56] | |||||||||||
| 66 | Dysprosi | Dy | +2 | +3 | +4 | [57] | ||||||||||||
| 67 | Holmi | Ho | +2 | +3 | [56] | |||||||||||||
| 68 | Erbi | Er | +2 | +3 | [56] | |||||||||||||
| 69 | Thuli | Tm | +2 | +3 | ||||||||||||||
| 70 | Ytterbi | Yb | +2 | +3 | ||||||||||||||
| 71 | Luteti | Lu | +2 | +3 | [56] | |||||||||||||
| 72 | Hafni | −2 | Hf | +1 | +2 | +3 | +4 | 4 | [15][58] | |||||||||
| 73 | Tantali | −3 | −1 | Ta | +1 | +2 | +3 | +4 | +5 | 5 | [15][34] | |||||||
| 74 | Wolfram | −4 | −2 | −1 | W | +1 | +2 | +3 | +4 | +5 | +6 | 6 | [15] | |||||
| 75 | Rheni | −3 | −1 | Re | +1 | +2 | +3 | +4 | +5 | +6 | +7 | 7 | ||||||
| 76 | Osmi | −4 | −2 | −1 | Os | +1 | +2 | +3 | +4 | +5 | +6 | +7 | +8 | 8 | [19][59] | |||
| 77 | Iridi | −3 | −1 | Ir | +1 | +2 | +3 | +4 | +5 | +6 | +7 | +8 | +9 | 9 | [60][61][62][63] | |||
| 78 | Platin | −3 | −2 | −1 | Pt | +1 | +2 | +3 | +4 | +5 | +6 | 10 | [19][64][65] | |||||
| 79 | Vàng | −3 | −2 | −1 | Au | +1 | +2 | +3 | +5 | 11 | [19] | |||||||
| 80 | Thủy ngân | −2 | Hg | +1 | +2 | 12 | [19][66] | |||||||||||
| 81 | Tali | −5 | −2 | −1 | Tl | +1 | +2 | +3 | 13 | [8][67][68][69] | ||||||||
| 82 | Chì | −4 | −2 | −1 | Pb | +1 | +2 | +3 | +4 | 14 | [8][70][71] | |||||||
| 83 | Bismuth | −3 | −2 | −1 | Bi | +1 | +2 | +3 | +4 | +5 | 15 | [72][73][74][75] | ||||||
| 84 | Poloni | −2 | Po | +2 | +4 | +5 | +6 | 16 | [76] | |||||||||
| 85 | Astatin | −1 | At | +1 | +3 | +5 | +7 | 17 | ||||||||||
| 86 | Radon | Rn | +2 | +6 | 18 | [77][78][79] | ||||||||||||
| 87 | Franci | Fr | +1 | 1 | ||||||||||||||
| 88 | Radi | Ra | +2 | 2 | ||||||||||||||
| 89 | Actini | Ac | +3 | 3 | ||||||||||||||
| 90 | Thori | Th | +1 | +2 | +3 | +4 | [80][81] | |||||||||||
| 91 | Protactini | Pa | +3 | +4 | +5 | |||||||||||||
| 92 | Urani | U | +1 | +2 | +3 | +4 | +5 | +6 | [82][83] | |||||||||
| 93 | Neptuni | Np | +2 | +3 | +4 | +5 | +6 | +7 | [84] | |||||||||
| 94 | Plutoni | Pu | +2 | +3 | +4 | +5 | +6 | +7 | [85] | |||||||||
| 95 | Americi | Am | +2 | +3 | +4 | +5 | +6 | +7 | [86] | |||||||||
| 96 | Curi | Cm | +3 | +4 | +6 | [87][88] | ||||||||||||
| 97 | Berkeli | Bk | +3 | +4 | ||||||||||||||
| 98 | Californi | Cf | +2 | +3 | +4 | |||||||||||||
| 99 | Einsteini | Es | +2 | +3 | +4 | [89] | ||||||||||||
| 100 | Fermi | Fm | +2 | +3 | ||||||||||||||
| 101 | Mendelevi | Md | +2 | +3 | ||||||||||||||
| 102 | Nobeli | No | +2 | +3 | ||||||||||||||
| 103 | Lawrenci | Lr | +3 | |||||||||||||||
| 104 | Rutherfordi | Rf | +4 | 4 | ||||||||||||||
| 105 | Dubni | Db | +5 | 5 | [90] | |||||||||||||
| 106 | Seaborgi | Sg | +6 | 6 | [91] | |||||||||||||
| 107 | Bohri | Bh | +7 | 7 | [92] | |||||||||||||
| 108 | Hassi | Hs | +8 | 8 | [93] | |||||||||||||
| 109 | Meitneri | Mt | 9 | |||||||||||||||
| 110 | Darmstadti | Ds | 10 | |||||||||||||||
| 111 | Roentgeni | Rg | 11 | |||||||||||||||
| 112 | Copernici | Cn | +2 | 12 | [94] | |||||||||||||
| 113 | Nihoni | Nh | 13 | |||||||||||||||
| 114 | Flerovi | Fl | 14 | |||||||||||||||
| 115 | Moscovi | Mc | 15 | |||||||||||||||
| 116 | Livermori | Lv | 16 | |||||||||||||||
| 117 | Tennessine | Ts | 17 | |||||||||||||||
| 118 | Oganesson | Og | 18 | |||||||||||||||
Một hình với một định dạng tương tự (được hiển thị dưới đây) đã được Irving Langmuir sử dụng vào năm 1919 trong một trong những bài báo ban đầu về quy tắc octet [95]. Sự tuần hoàn của các trạng thái oxy hóa là một trong những bằng chứng cho thấy Langmuir có thể áp dụng quy luật này.
Tham khảo
[sửa | sửa mã nguồn]- ^ a b Greenwood, Norman N.; Earnshaw, A. (1997), Chemistry of the Elements (ấn bản thứ 2), Oxford: Butterworth-Heinemann, tr. 27–28, ISBN 0-7506-3365-4
- ^ a b c d e Na(−1), K(−1), Rb(−1), and Cs(−1) are known in alkalides; the table by Greenwood and Earnshaw shows −1 only for Na and also erroneously for Li; no lithides are described.
- ^ Be(I) has been observed in beryllium monohydride (BeH); see Shayesteh, A.; Tereszchuk, K.; Bernath, P. F.; Colin, R. (2003). “Infrared Emission Spectra of BeH and BeD” (PDF). J. Chem. Phys. bernath.uwaterloo.ca. 118 (3): 1158. doi:10.1063/1.1528606. Bản gốc (PDF) lưu trữ ngày 2 tháng 12 năm 2007. Truy cập ngày 10 tháng 12 năm 2007.
- ^ B(−1) has been observed in magie diboride (MgB2), see James Keeler, Peter Wothers (2014). Chemical Structure and Reactivity: An Integrated Approach. Oxford University Press.Quản lý CS1: sử dụng tham số tác giả (liên kết)
- ^ B(−5) has been observed in Al3BC, see Melanie Schroeder. “Eigenschaften von borreichen Boriden und Scandium-Aluminium-Oxid-Carbiden” (PDF) (bằng tiếng Đức). tr. 139. Bản gốc (PDF) lưu trữ ngày 2 tháng 4 năm 2015. Truy cập ngày 3 tháng 9 năm 2017.
- ^ Low valent magnesium compounds with Mg(I) have been obtained using bulky ligands; see Green, S. P.; Jones C.; Stasch A. (tháng 12 năm 2007). “Stable Magnesium(I) Compounds with Mg-Mg Bonds”. Science. 318 (5857): 1754–1757. Bibcode:2007Sci...318.1754G. doi:10.1126/science.1150856. PMID 17991827.
- ^ Al(II) has been observed in aluminium(II) oxit (AlO); see D. C. Tyte (1964). “Red (B2Π–A2σ) Band System of Aluminium Monoxide”. Nature. 202 (4930): 383–384. Bibcode:1964Natur.202..383T. doi:10.1038/202383a0., and in dialanes (R2Al—AlR2); see Uhl, Werner "Organoelement Compounds Possessing Al—Al, Ga—Ga, In—In, and Tl—Tl Single Bonds" Advances in Organometallic Chemistry Volume 51, 2004, Pages 53–108. doi:10.1016/S0065-3055(03)51002-4
- ^ a b c d e f g h i Negative oxidation states of p-block metals (Al, Ga, In, Sn, Tl, Pb, Bi, Po) and metalloids (Si, Ge, As, Sb, Te, At) may occur in Zintl phases, see: [1], p. 259 and [2] (both in German).
- ^ Al(−2) has been observed in Sr14[Al4]2[Ge]3, see Wemdorff, Marco; Röhr, Caroline (2007). “Sr14[Al4]2[Ge]3: Eine Zintl-Phase mit isolierten [Ge]4–- und [Al4]8–-Anionen / Sr14[Al4]2[Ge]3: A Zintl Phase with Isolated [Ge]4–- and [Al4]8– Anions”. Zeitschrift für Naturforschung B (bằng tiếng Đức). 62 (10): 1227. doi:10.1515/znb-2007-1001.
- ^ The equilibrium Cl2O6⇌2ClO3 is mentioned by Greenwood and Earnshaw, but it has been refuted, see Lopez, Maria; Juan E. Sicre (1990). “Physicochemical properties of chlorine oxides. 1. Composition, ultraviolet spectrum, and kinetics of the thermolysis of gaseous dichlorine hexoxide”. J. Phys. Chem. 94 (9): 3860–3863. doi:10.1021/j100372a094., and Cl2O6 is actually chlorine(V,VII) oxide. However, ClO3 has been observed, see Grothe, Hinrich; Willner, Helge (1994). “Chlorine Trioxide: Spectroscopic Properties, Molecular Structure, and Photochemical Behavior”. Angew. Chem. Int. Ed. 33 (14): 1482–1484. doi:10.1002/anie.199414821.
- ^ Ca(I) has been observed; see Krieck, Sven; Görls, Helmar; Westerhausen, Matthias (2010). “Mechanistic Elucidation of the Formation of the Inverse Ca(I) Sandwich Complex [(thf)3Ca(μ-C6H3-1,3,5-Ph3)Ca(thf)3] and Stability of Aryl-Substituted Phenylcalcium Complexes”. Journal of the American Chemical Society. 132 (35): 12492–501. doi:10.1021/ja105534w. PMID 20718434.
- ^ Sc(I) has been observed; see Polly L. Arnold; F. Geoffrey; N. Cloke; Peter B. Hitchcock & John F. Nixon (1996). “The First Example of a Formal Scandium(I) Complex: Synthesis and Molecular Structure of a 22-Electron Scandium Triple Decker Incorporating the Novel 1,3,5-Triphosphabenzene Ring”. J. Am. Chem. Soc. 118 (32): 7630–7631. doi:10.1021/ja961253o.
- ^ Sc(II) has been observed; see Woen, David H.; Chen, Guo P.; Ziller, Joseph W.; Boyle, Timothy J.; Furche, Filipp; Evans, William J. (tháng 1 năm 2017). “Solution Synthesis, Structure, and CO Reduction Reactivity of a Scandium(II) Complex”. Angewandte Chemie International Edition. 56: 2050–2053. doi:10.1002/anie.201611758.
- ^ Ti(I) has been observed in [Ti(η6-1,3,5-C6H3iPr3)2][BAr4] (Ar = C6H5, p-C6H4F, 3,5-C6H3(CF3)2); see Fausto Calderazzo, Isabella Ferri, Guido Pampaloni, Ulli Englert, Malcolm L. H. Green (1997). “Synthesis of [Ti(η6-1,3,5-C6H3iPr3)2][BAr4] (Ar = C6H5, p-C6H4F, 3,5-C6H3(CF3)2), the First Titanium(I) Derivatives”. Organometallics. 16 (14): 3100–3101. doi:10.1021/om970155o.Quản lý CS1: sử dụng tham số tác giả (liên kết)
- ^ a b c d e f g h i j k l Ti(−2), V(−3), Cr(−4), Co(−3), Zr(−2), Nb(−3), Mo(−4), Ru(−2), Rh(−3), Hf(−2), Ta(−3), and W(−4) occur in anionic binary metal carbonyls; see [3], p. 4 (in German); [4], pp. 97–100; [5], p. 239
- ^ Ti(−1) has been reported in [Ti(bipy)3]−, but was later shown to be Ti(+3); see Bowman, A. C.; England, J.; Sprouls, S.; Weihemüller, T.; Wieghardt, K. (2013). “Electronic structures of homoleptic [tris(2,2'-bipyridine)M]n complexes of the early transition metals (M = Sc, Y, Ti, Zr, Hf, V, Nb, Ta; n = 1+, 0, 1-, 2-, 3-): an experimental and density functional theoretical study”. Inorganic Chemistry. 52 (4): 2242–56. doi:10.1021/ic302799s. PMID 23387926. However, Ti(−1) occurs in [Ti(η-C6H6]− and [Ti(η-C6H5CH3)]−, see Bandy, J. A.; Berry, A.; Green, M. L. H.; Perutz, R. N.; Prout, K.; Verpeautz, J.-N. (1984). “Synthesis of anionic sandwich compounds: [Ti(η-C6H5R)2]– and the crystal structure of [K(18-crown-6)(µ-H)Mo(η-C5H5)2]”. Inorganic Chemistry. 52 (4): 729–731. doi:10.1039/C39840000729.
- ^ Fe(VII) has been observed in [FeO4]−; see Lu, Jun-Bo; Jian, Jiwen; Huang, Wei; Lin, Hailu; Zhou, Mingfei (2016). “Experimental and theoretical identification of the Fe(VII) oxidation state in FeO4−”. Physical Chemistry Chemical Physics. 18: 31125–31131. doi:10.1039/C6CP06753K.
- ^ Fe(VIII) has been reported; see Yurii D. Perfiliev; Virender K. Sharma (2008). “Higher Oxidation States of Iron in Solid State: Synthesis and Their Mössbauer Characterization – Ferrates – ACS Symposium Series (ACS Publications)”. Platinum Metals Review. 48 (4): 157–158. doi:10.1595/147106704X10801. However, its existence has been disputed.
- ^ a b c d e f g h i j Fe(−4), Ru(−4), and Os(−4) have been observed in metal-rich compounds containing octahedral complexes [MIn6−xSnx]; Pt(−3) (as a dimeric anion [Pt–Pt]6−), Cu(−2), Zn(−2), Ag(−2), Cd(−2), Au(−2), and Hg(−2) have been observed (as dimeric and monomeric anions; dimeric ions were initially reported to be [T–T]2− for Zn, Cd, Hg, but later shown to be [T–T]4− for all these elements) in La2Pt2In, La2Cu2In, Ca5Au3, Ca5Ag3, Ca5Hg3, Sr5Cd3, Ca5Zn3(structure (AE2+)5(T–T)4−T2−⋅4e−), Yb3Ag2, Ca5Au4, and Ca3Hg2; Au(–3) has been observed in ScAuSn and in other 18-electron half-Heusler compounds. See Changhoon Lee; Myung-Hwan Whangbo (2008). “Late transition metal anions acting as p-metal elements”. Frontiers in Solid State Chemistry. 10 (4): 444–449. doi:10.1016/j.solidstatesciences.2007.12.001. and Changhoon Lee; Myung-Hwan Whangbo; Jürgen Köhler (2010). “Analysis of Electronic Structures and Chemical Bonding of Metal-rich Compounds. 2. Presence of Dimer (T–T)4– and Isolated T2– Anions in the Polar Intermetallic Cr5B3-Type Compounds AE5T3 (AE = Ca, Sr; T = Au, Ag, Hg, Cd, Zn)”. ZAAC. 636 (1): 36–40. doi:10.1002/zaac.200900421.
- ^ Ni(−2) has been observed in Li2[Ni(1,5-COD)2], see Jonas, Klaus (1975). “Dilithium-Nickel-Olefin Complexes. Novel Bimetal Complexes Containing a Transition Metal and a Main Group Metal”. Angew. Chem. Int. Ed. 14 (11): 752–753. doi:10.1002/anie.197507521. and Ellis, John E. (2006). “Adventures with Substances Containing Metals in Negative Oxidation States”. Inorganic Chemistry. 45 (8): 3167–86. doi:10.1021/ic052110i.
- ^ Zn(I) has been observed in decamethyldizincocene (Zn2(η5–C5Me5)2); see Resa, I.; Carmona, E.; Gutierrez-Puebla, E.; Monge, A. (2004). “Decamethyldizincocene, a Stable Compound of Zn(I) with a Zn-Zn Bond”. Science. 305 (5687): 1136–8. doi:10.1126/science.1101356. PMID 15326350.
- ^ Ga(−2), Ga(−4), and Ga(−5) have been observed in the magnesium gallides MgGa, Mg2Ga, and Mg5Ga2, respectively; see Patrick Hofmann. “Colture. Ein Programm zur interaktiven Visualisierung von Festkörperstrukturen sowie Synthese, Struktur und Eigenschaften von binären und ternären Alkali- und Erdalkalimetallgalliden” (PDF) (bằng tiếng Đức). tr. 72.
- ^ Ge(−1), Ge(−2), and Ge(−3) have been observed in germanides; see Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (1995). “Germanium”. Lehrbuch der Anorganischen Chemie (bằng tiếng Đức) (ấn bản thứ 101). Walter de Gruyter. tr. 953–959. ISBN 3-11-012641-9..
- ^ As(I) has been observed in arsenic(I) iodide (AsI); see Ellis, Bobby D.; MacDonald, Charles L. B. (2004). “Stabilized Arsenic(I) Iodide: A Ready Source of Arsenic Iodide Fragments and a Useful Reagent for the Generation of Clusters”. Inorganic Chemistry. 43 (19): 5981–6. doi:10.1021/ic049281s. PMID 15360247.
- ^ As(IV) has been observed in arsenic(IV) hydroxide (As(OH)4) and HAsO−
3; see Kläning, Ulrik K.; Bielski, Benon H. J.; Sehested, K. (1989). “Arsenic(IV). A pulse-radiolysis study”. Inorganic Chemistry. 28 (14): 2717–24. doi:10.1021/ic00313a007. - ^ Se(−1) has been observed in diselenides(2−) (Se22−).
- ^ Se(I) has been observed in selenium(I) chloride (Se2Cl2); see “Selenium: Selenium(I) chloride compound data”. WebElements.com. Truy cập ngày 10 tháng 12 năm 2007.
- ^ Se(III) has been observed in Se2NBr3; see Lau, Carsten; Neumüller, Bernhard; Vyboishchikov, Sergei F.; Frenking, Gernot; Dehnicke, Kurt; Hiller, Wolfgang; Herker, Martin (1996). “Se2NBr3, Se2NCl5, Se2NCl−6: New Nitride Halides of Selenium(III) and Selenium(IV)”. Chemistry: A European Journal. 2: 1393–1396. doi:10.1002/chem.19960021108.
- ^ Se(V) has been observed in SeO2−
3 and HSeO2−
4; see Kläning, Ulrik K.; Sehested, K. (1986). “Selenium(V). A pulse radiolysis study”. Inorganic Chemistry. 90 (21): 5460–4. doi:10.1021/j100412a112. - ^ Sr(I) has been observed in stronti monofluoride (SrF); see P. Colarusso; Guo, B.; Zhang, K.-Q.; Bernath, P.F.; và đồng nghiệp (1996). “High-Resolution Infrared Emission Spectrum of Strontium Monofluoride” (PDF). J. Molecular Spectroscopy. 175: 158–171. Bibcode:1996JMoSp.175..158C. doi:10.1006/jmsp.1996.0019. Bản gốc (PDF) lưu trữ ngày 8 tháng 3 năm 2012.
- ^ Y(I) has been observed in ytrium(I) bromide (YBr); see “Ytrium: ytrium(I) bromide compound data”. OpenMOPAC.net. Bản gốc lưu trữ ngày 23 tháng 7 năm 2011. Truy cập ngày 10 tháng 12 năm 2007.
- ^ Y(II) has been observed in [(18-crown-6)K][(C5H4SiMe3)3Y]; see MacDonald, M. R.; Ziller, J. W.; Evans, W. J. (2011). “Synthesis of a Crystalline Molecular Complex of Y2+, [(18-crown-6)K][(C5H4SiMe3)3Y]”. J. Am. Chem. Soc. 133 (40): 15914–17. doi:10.1021/ja207151y.
- ^ Zr(−1) has been reported in [Zr(bipy)3]− (see Greenwood, Norman N.; Earnshaw, A. (1997), Chemistry of the Elements (ấn bản thứ 2), Oxford: Butterworth-Heinemann, tr. 960, ISBN 0-7506-3365-4 and Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (1995). “Zirconium”. Lehrbuch der Anorganischen Chemie (bằng tiếng Đức) (ấn bản thứ 101). Walter de Gruyter. tr. 1413. ISBN 3-11-012641-9.), but was later shown to be Zr(+4); see Bowman, A. C.; England, J.; Sprouls, S.; Weihemüller, T.; Wieghardt, K. (2013). “Electronic structures of homoleptic [tris(2,2'-bipyridine)M]n complexes of the early transition metals (M = Sc, Y, Ti, Zr, Hf, V, Nb, Ta; n = 1+, 0, 1-, 2-, 3-): an experimental and density functional theoretical study”. Inorganic Chemistry. 52 (4): 2242–56. doi:10.1021/ic302799s. PMID 23387926.
- ^ a b Nb(I) and Ta(I) occur in CpNb(CO)4 and CpTa(CO)4, see Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (1995). “Tantal”. Lehrbuch der Anorganischen Chemie (bằng tiếng Đức) (ấn bản thứ 101). Walter de Gruyter. tr. 1430. ISBN 3-11-012641-9. and King, R. Bruce (1969). Transition-Metal Organometallic Chemistry: An Introduction. Academic Press. tr. 11. ISBN 0-32-315996-6.
- ^ Pd(I) has been observed; see Crabtree, R. H. (2002). “CHEMISTRY: A New Oxidation State for Pd?”. Science. 295 (5553): 288–289. doi:10.1126/science.1067921.
- ^ Pd(III) has been observed; see Powers, D. C.; Ritter, T. (2011). “Palladium(III) in Synthesis and Catalysis” (PDF). Top. Organomet. Chem. Topics in Organometallic Chemistry. 35: 129–156. doi:10.1007/978-3-642-17429-2_6. ISBN 978-3-642-17428-5. Lưu trữ bản gốc ngày 12 tháng 6 năm 2013.Quản lý CS1: URL hỏng (liên kết)
- ^ The Ag− ion has been observed in metal ammonia solutions: see Tran, N. E.; Lagowski, J. J. (2001). “Metal Ammonia Solutions: Solutions Containing Argentide Ions”. Inorganic Chemistry. 40 (5): 1067–68. doi:10.1021/ic000333x.
- ^ Cd(I) has been observed in cadmium(I) tetrachloroaluminate (Cd2(AlCl4)2); see Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (1985). “Cadmium”. Lehrbuch der Anorganischen Chemie (bằng tiếng Đức) . Walter de Gruyter. tr. 1056–1057. ISBN 3-11-007511-3.
- ^ In(–5) has been observed in La3InGe, see Guloy, A. M.; Corbett, J. D. (1996). “Synthesis, Structure, and Bonding of Two Lanthanum Indium Germanides with Novel Structures and Properties”. Inorganic Chemistry. 35 (9): 2616–22. doi:10.1021/ic951378e.
- ^ In(−2) has been observed in Na2In, see [6], p. 69.
- ^ Sn(−3) has been observed in [Sn2]6−, e.g. in (Ba2)4+(Mg4)8+Sn4−(Sn2)6−Sn2− (with square (Sn2−)n sheets), see Papoian, Garegin A.; Hoffmann, Roald (2000). “Hypervalent Bonding in One, Two, and Three Dimensions: Extending the Zintl–Klemm Concept to Nonclassical Electron-Rich Networks” (PDF). Angew. Chem. Int. Ed. 2000 (39): 2408–2448. doi:10.1002/1521-3773(20000717)39:14<2408::aid-anie2408>3.0.co;2-u. Truy cập ngày 23 tháng 2 năm 2015.
- ^ Sn(I) and Sn(III) have been observed in organotin compounds
- ^ Sb(−2) has been observed in [Sb2]4−, e.g. in RbBa4[Sb2][Sb][O], see Boss, Michael; Petri, Denis; Pickhard, Frank; Zönnchen, Peter; Röhr, Caroline (2005). “Neue Barium-Antimonid-Oxide mit den Zintl-Ionen [Sb]3−, [Sb2]4− und 1∞[Sbn]n− / New Barium Antimonide Oxides containing Zintl Ions [Sb]3−, [Sb2]4− and 1∞[Sbn]n−”. Zeitschrift für anorganische und allgemeine Chemie (bằng tiếng Đức). 631 (6–7): 1181–1190. doi:10.1002/zaac.200400546. Truy cập ngày 23 tháng 2 năm 2015.
- ^ Sb(I) and Sb(II) have been observed in organoantimony compounds; for Sb(I), see Šimon, Petr; de Proft, Frank; Jambor, Roman; Růžička, Aleš; Dostál, Libor (2010). “Monomeric Organoantimony(I) and Organobismuth(I) Compounds Stabilized by an NCN Chelating Ligand: Syntheses and Structures”. Angewandte Chemie International Edition. 49 (32): 5468–5471. doi:10.1002/anie.201002209. PMID 20602393. Truy cập ngày 23 tháng 2 năm 2015.
- ^ Sb(IV) has been observed in [SbCl
6]2−
, see Nobuyoshi Shinohara; Masaaki Ohsima (2000). “Production of Sb(IV) Chloro Complex by Flash Photolysis of the Corresponding Sb(III) and Sb(V) Complexes in CH3CN and CHCl3”. Bulletin of the Chemical Society of Japan. 73 (7): 1599–1604. doi:10.1246/bcsj.73.1599. - ^ Te(I) has been observed in tellurium iodide (TeI), see “Tellurium: tellurium iodide”. WebElements.com. Truy cập ngày 23 tháng 2 năm 2015.
- ^ Te(III) has been observed in [Te(N(SiMe3)2)2]+, see Heinze, Thorsten; Roesky, Herbert W.; Pauer, Frank; Stalke, Dietmar; Sheldrick, George M. (1991). “Synthesis and Structure of the First Tellurium(III) Radical Cation”. Angewandte Chemie International Edition. 30 (12): 1678. doi:10.1002/anie.199116771. Truy cập ngày 23 tháng 2 năm 2015..
- ^ Te(V) is mentioned by Greenwood and Earnshaw, but they do not give any example of a Te(V) compound. What was long thought to be ditellurium decafluoride (Te2F10) is actually bis(pentafluorotelluryl) oxide, F5TeOTeF5: see Watkins, P. M. (1974). “Ditellurium decafluoride - A Continuing Myth”. Journal of Chemical Education. 51 (9): 520–521. doi:10.1021/ed051p520. However, Te(V) has been observed in H
2TeO−
4, TeO−
3, HTeO2−
4, and TeO3−
4; see Kläning, Ulrik K.; Sehested, K. (2001). “Tellurium(V). A Pulse Radiolysis Study”. The Journal of Physical Chemistry A. 105 (27): 6637–45. doi:10.1021/jp010577i. - ^ I(IV) has been observed in iodine dioxide (IO2); see Pauling, Linus (1988). “Oxygen Compounds of Nonmetallic Elements”. General Chemistry (ấn bản thứ 3). Dover Publications, Inc. tr. 259. ISBN 0-486-65622-5.
- ^ I(VI) has been observed in IO3, IO42−, H5IO6−, H2IO52−, H4IO62−, and HIO53−; see Kläning, Ulrik K.; Sehested, Knud; Wolff, Thomas (1981). “Laser flash photolysis and pulse radiolysis of iodate and periodate in aqueous solution. Properties of iodine(VI)”. J. Chem. Soc., Faraday Trans. 1. 77: 1707–18. doi:10.1039/F19817701707.
- ^ Xe(I) has been reported in xenon hexafluoroplatinate và xenon hexafluororhodate (see Pauling, Linus (1988). General Chemistry (ấn bản thứ 3). Dover Publications, Inc. tr. 250. ISBN 0-486-65622-5.), however these compounds were later found to contain Xe(II).
- ^ Ba(I) has been observed in bari monofluoride (BaF); see P. Colarusso; Guo, B.; Zhang, K.-Q.; Bernath, P.F.; và đồng nghiệp (1995). “High-Resolution Fourier Transform Infrared Emission Spectrum of Barium Monofluoride” (PDF). J. Molecular Spectroscopy. 170: 59. Bibcode:1996JMoSp.175..158C. doi:10.1006/jmsp.1996.0019. Bản gốc (PDF) lưu trữ ngày 10 tháng 3 năm 2005.
- ^ La(I) has been observed in lanthanum monohydride (LaH); see Ram, R. S.; Bernath, P. F. (1996). “Fourier Transform Emission Spectroscopy of New Infrared Systems of LaH and LaD” (PDF). J. Molecular Spectroscopy. 104: 6444. doi:10.1063/1.471365. Bản gốc (PDF) lưu trữ ngày 10 tháng 3 năm 2005.
- ^ Pr(V) has been observed in [PrO2]+; see Zhang, Qingnan; Hu, Shu-Xian; Qu, Hui; Su, Jing; Wang, Guanjun; Lu, Jun-Bo; Chen, Mohua; Zhou, Mingfei; Li, Jun (ngày 6 tháng 6 năm 2016). “Pentavalent Lanthanide Compounds: Formation and Characterization of Praseodymium(V) Oxides”. Angewandte Chemie International Edition. 55 (24): 6896–6900. doi:10.1002/anie.201602196. ISSN 1521-3773. PMID 27100273.
- ^ Nd(IV) has been observed in unstable solid state compounds; see Bản mẫu:Holleman&Wiberg
- ^ a b c d e All the lanthanides (La–Lu) in the +2 oxidation state have been observed (except La, Gd, Lu) in dilute, solid solutions of dihalides of these elements in alkaline earth dihalides (see Bản mẫu:Holleman&Wiberg) and (except Pm) in organometallic molecular complexes, see Lanthanides Topple Assumptions and Meyer, G. (2014). “All the Lanthanides Do It and Even Uranium Does Oxidation State +2”. Angewandte Chemie International Edition. 53 (14): 3550–51. doi:10.1002/anie.201311325. PMID 24616202.. Additionally, all the lanthanides (La–Lu) form dihydrides (LnH2), dicarbides (LnC2), monosulfides (LnS), monoselenides (LnSe), and monotellurides (LnTe), but for most elements these compounds have Ln3+ ions with electrons delocalized into conduction bands, e. g. Ln3+(H−)2(e−).
- ^ Dy(IV) has been observed in unstable solid state compounds; see Bản mẫu:Holleman&Wiberg
- ^ Hf(I) has been observed in hafnium monobromide (HfBr), see Marek, G.S.; Troyanov, S.I.; Tsirel'nikov, V.I. (1979). “Кристаллическое строение и термодинамические характеристики монобромидов циркония и гафния / Crystal structure and thermodynamic characteristics of monobromides of zirconium and hafnium”. Журнал неорганической химии / Russian Journal of Inorganic Chemistry (bằng tiếng Nga). 24 (4): 890–893.
- ^ Os(−1) has been observed in Na
2[Os
4(CO)
13]; see Krause, J.; Siriwardane, Upali; Salupo, Terese A.; Wermer, Joseph R.; Knoeppel, David W.; Shore, Sheldon G. (1993). “Preparation of [Os3(CO)11]2− and its reactions with Os3(CO)12; structures of [Et4N] [HOs3(CO)11] and H2OsS4(CO)”. Journal of Organometallic Chemistry. 454: 263–271. doi:10.1016/0022-328X(93)83250-Y. and Carter, Willie J.; Kelland, John W.; Okrasinski, Stanley J.; Warner, Keith E.; Norton, Jack R. (1982). “Mononuclear hydrido alkyl carbonyl complexes of osmium and their polynuclear derivatives”. Inorganic Chemistry. 21 (11): 3955–3960. doi:10.1021/ic00141a019. - ^ Ir(−3) has been observed in Ir(CO)33−; see Greenwood, Norman N.; Earnshaw, A. (1997), Chemistry of the Elements (ấn bản thứ 2), Oxford: Butterworth-Heinemann, tr. 1117, ISBN 0-7506-3365-4
- ^ Ir(VII) has been observed in [(η2-O2)IrO2]+; see C&EN: Iridium dressed to the nines Lưu trữ 2015-03-09 tại Wayback Machine.
- ^ Ir(VIII) has been observed in iridium tetroxit (IrO4); see Gong, Yu; Zhou, Mingfei; Kaupp, Martin; Riedel, Sebastian (2009). “Formation and Characterization of the Iridium Tetroxide Molecule with Iridium in the Oxidation State +VIII”. Angewandte Chemie International Edition. 48 (42): 7879–7883. doi:10.1002/anie.200902733.
- ^ Ir(IX) has been observed in IrO+
4; see Wang, Guanjun; Zhou, Mingfei; Goettel, James T.; Schrobilgen, Gary G.; Su, Jing; Li, Jun; Schlöder, Tobias; Riedel, Sebastian (ngày 21 tháng 8 năm 2014). “Identification of an iridium-containing compound with a formal oxidation state of IX”. Nature. 514: 475–477. doi:10.1038/nature13795. PMID 25341786. - ^ Pt(−1) and Pt(−2) have been observed in the bari platinides Ba2Pt and BaPt, respectively: see Karpov, Andrey; Konuma, Mitsuharu; Jansen, Martin (2006). “An experimental proof for negative oxidation states of platinum: ESCA-measurements on barium platinides”. Chemical Communications (8): 838–840. doi:10.1039/b514631c. PMID 16479284.
- ^ Pt(I) and Pt(III) have been observed in bimetallic and polymetallic species; see Kauffman, George B.; Thurner, Joseph J.; Zatko, David A. (1967). “Ammonium Hexachloroplatinate(IV)”. Inorganic Syntheses. Inorganic Syntheses. 9: 182–185. doi:10.1002/9780470132401.ch51. ISBN 978-0-470-13240-1.
- ^ Hg(IV) has been reported in mercury(IV) fluoride (HgF4); see Xuefang Wang; Lester Andrews; Sebastian Riedel; Martin Kaupp (2007). “Mercury Is a Transition Metal: The First Experimental Evidence for HgF4”. Angew. Chem. Int. Ed. 46 (44): 8371–8375. doi:10.1002/anie.200703710. PMID 17899620. However, it could not be confirmed by later experiments; see Is mercury a transition metal? Lưu trữ 2016-10-12 tại Wayback Machine
- ^ Tl(−5) has been observed in Na23K9Tl15.3, see Dong, Z.-C.; Corbett, J. D. (1996). “Na23K9Tl15.3: An Unusual Zintl Compound Containing Apparent Tl57−, Tl48−, Tl37−, and Tl5− Anions”. Inorganic Chemistry. 35 (11): 3107–12. doi:10.1021/ic960014z.
- ^ Tl(−1) has been observed in caesi thallide (CsTl); see King, R. B.; Schleyer, R. (2004). “Theory and concepts in main-group cluster chemistry”. Trong Driess, M.; Nöth, H. (biên tập). Molecular clusters of the main group elements. Wiley-VCH, Chichester. tr. 19. ISBN 978-3-527-61437-0.
- ^ Tl(+2) has been observed in tetrakis(hypersilyl)dithallium ([(Me3Si)Si]2Tl—Tl[Si(SiMe3)]2), see Sonja Henkel; Dr. Karl Wilhelm Klinkhammer; Dr. Wolfgang Schwarz (1994). “Tetrakis(hypersilyl)dithallium(Tl—Tl): A Divalent Thallium Compound”. Angew. Chem. Int. Ed. 33 (6): 681–683. doi:10.1002/anie.199406811..
- ^ Pb(−2) has been observed in BaPb, see Ferro, Riccardo (2008). Nicholas C. Norman (biên tập). Intermetallic Chemistry. Elsevier. tr. 505. ISBN 978-0-08-044099-6. and Todorov, Iliya; Sevov, Slavi C. (2004). “Heavy-Metal Aromatic Rings: Cyclopentadienyl Anion Analogues Sn56− and Pb56− in the Zintl Phases Na8BaPb6, Na8BaSn6, and Na8EuSn6”. Inorganic Chemistry. 43 (20): 6490–94. doi:10.1021/ic000333x.
- ^ Pb(+1) and Pb(+3) have been observed in organolead compounds, e.g. hexamethyldiplumbane Pb2(CH3)6; for Pb(I), see Siew-Peng Chia; Hong-Wei Xi; Yongxin Li; Kok Hwa Lim; Cheuk-Wai So (2013). “A Base-Stabilized Lead(I) Dimer and an Aromatic Plumbylidenide Anion”. Angew. Chem. Int. Ed. 52 (24): 6298–6301. doi:10.1002/anie.201301954..
- ^ Bi(−2) and Bi(−1) occur in Zintl phases, e.g. (Ca2+)22[Bi4]4−([Bi2]4−)4[Bi3−]8; see Ponou, Siméon (2006). “Germanides, Germanide-Tungstate Double Salts and Substitution Effects in Zintl Phases”. Technische Universität München. Lehrstuhl für Anorganische Chemie mit Schwerpunkt Neue Materialien. tr. 68.
- ^ Bi(I) has been observed in bismuth monobromide (BiBr) and bismuth monoiodide (BiI); see Godfrey, S. M.; McAuliffe, C. A.; Mackie, A. G.; Pritchard, R. G. (1998). Nicholas C. Norman (biên tập). Chemistry of arsenic, antimony, and bismuth. Springer. tr. 67–84. ISBN 0-7514-0389-X.
- ^ Bi(+2) has been observed in dibismuthines (R2Bi—BiR2), see Arthur J. Ashe III (1990). “Thermochromic Distibines and Dibismuthines”. Advances in Organometallic Chemistry. 30: 77–97. doi:10.1016/S0065-3055(08)60499-2.
- ^ Bi(IV) has been observed; see A. I. Aleksandrov, I. E. Makarov (1987). “Formation of Bi(II) and Bi(IV) in aqueous hydrochloric solutions of Bi(III)”. Bulletin of the Academy of Sciences of the USSR, Division of chemical science. 36 (2): 217–220. doi:10.1007/BF00959349.
- ^ Po(V) has been observed in dioxidopolonium(1+) (PoO+
2); see Thayer, John S. (2010). Relativistic Effects and the Chemistry of the Heavier Main Group Elements. tr. 78. doi:10.1007/978-1-4020-9975-5_2. - ^ Rn(II) has been observed in radon difluoride (RnF2); see Stein, L. (1970). “Ionic Radon Solution”. Science. 168 (3929): 362–4. Bibcode:1970Sci...168..362S. doi:10.1126/science.168.3929.362. PMID 17809133. and Kenneth S. Pitzer (1975). “Fluorides of radon and element 118”. J. Chem. Soc., Chem. Commun. (18): 760b–761. doi:10.1039/C3975000760b.
- ^ Rn(IV) is reported by Greenwood and Earnshaw, but is not known to exist; see Sykes, A. G. (1998). “Recent Advances in Noble-Gas Chemistry”. Advances in Inorganic Chemistry. 46. Academic Press. tr. 91–93. ISBN 978-0-12-023646-6. Truy cập ngày 22 tháng 11 năm 2012.
- ^ Rn(VI) is known in radon trioxit (RnO3); see Sykes, A. G. (1998). “Recent Advances in Noble-Gas Chemistry”. Advances in Inorganic Chemistry. 46. Academic Press. tr. 91–93. ISBN 978-0-12-023646-6. Truy cập ngày 22 tháng 11 năm 2012.
- ^ Th(I) is known in thorium(I) bromide (ThBr); see Wickleder, Mathias S.; Fourest, Blandine; Dorhout, Peter K. (2006). “Thorium”. Trong Morss, Lester R.; Edelstein, Norman M.; Fuger, Jean (biên tập). The Chemistry of the Actinide and Transactinide Elements (PDF). 3 (ấn bản thứ 3). Dordrecht, the Netherlands: Springer. tr. 52–160. doi:10.1007/1-4020-3598-5_3. Bản gốc (PDF) lưu trữ ngày 7 tháng 3 năm 2016.
- ^ Th(II) and Th(III) are observed in [ThII{η5-C5H3(SiMe3)2}3]− and [ThIII{η5-C5H3(SiMe3)2}3], see Langeslay, Ryan R.; Fieser, Megan E.; Ziller, Joseph W.; Furche, Philip; Evans, William J. (2015). “Synthesis, structure, and reactivity of crystalline molecular complexes of the {[C5H3(SiMe3)2]3Th}1− anion containing thorium in the formal +2 oxidation state”. Chem. Sci. 6: 517–521. doi:10.1039/C4SC03033H. Truy cập ngày 16 tháng 7 năm 2016.
- ^ U(I) has been observed in uranium monofluoride (UF) and uranium monochloride (UCl), see Sykes, A. G. (1990). “Compounds of Thorium and Uranium”. Advances in Inorganic Chemistry. 34. Academic Press. tr. 87–88. ISBN 0-12-023634-6. Truy cập ngày 22 tháng 3 năm 2015.
- ^ U(II) has been observed in [K(2.2.2-Cryptand)][(C5H4SiMe3)3U], see MacDonald, Matthew R.; Fieser, Megan E.; Bates, Jefferson E.; Ziller, Joseph W.; Furche, Filipp; Evans, William J. (2013). “Identification of the +2 Oxidation State for Uranium in a Crystalline Molecular Complex, [K(2.2.2-Cryptand)][(C5H4SiMe3)3U]”. J. Am. Chem. Soc. 135 (36): 13310–13313. doi:10.1021/ja406791t.
|ngày truy cập=cần|url=(trợ giúp) - ^ Np(II) has been observed, see Dutkiewicz, Michał S.; Apostolidis, Christos; Walter, Olaf; Arnold, Polly L (2017). “Reduction chemistry of neptunium cyclopentadienide complexes: from structure to understanding”. Chem. Sci. 8: 2553–2561. doi:10.1039/C7SC00034K.
|ngày truy cập=cần|url=(trợ giúp) - ^ Pu(II) has been observed in {Pu[C5H3(SiMe3)2]3}−; see Windorff, Cory J.; Chen, Guo P; Cross, Justin N; Evans, William J.; Furche, Filipp; Gaunt, Andrew J.; Janicke, Michael T.; Kozimor, Stosh A.; Scott, Brian L. (2017). “Identification of the Formal +2 Oxidation State of Plutonium: Synthesis and Characterization of {PuII[C5H3(SiMe3)2]3}−”. J. Am. Chem. Soc. 139 (11): 3970–3973. doi:10.1021/jacs.7b00706.
- ^ Am(VII) has been observed in AmO5−
6; see Americium, Das Periodensystem der Elemente für den Schulgebrauch (The periodic table of elements for schools) chemie-master.de (in German), Retrieved ngày 28 tháng 11 năm 2010 and Greenwood, Norman N.; Earnshaw, A. (1997), Chemistry of the Elements (ấn bản thứ 2), Oxford: Butterworth-Heinemann, tr. 1265, ISBN 0-7506-3365-4 - ^ Cm(VI) has been observed in curium trioxit (CmO3) and dioxidocurium(2+) (CmO2+
2); see Domanov, V. P.; Lobanov, Yu. V. (tháng 10 năm 2011). “Formation of volatile curium(VI) trioxide CmO3”. Radiochemistry. SP MAIK Nauka/Interperiodica. 53 (5): 453–6. doi:10.1134/S1066362211050018. - ^ Cm(VIII) has been reported to possibly occur in curium tetroxit (CmO4); see Domanov, V. P. (tháng 1 năm 2013). “Possibility of generation of octavalent curium in the gas phase in the form of volatile tetraoxide CmO4”. Radiochemistry. SP MAIK Nauka/Interperiodica. 55 (1): 46–51. doi:10.1134/S1066362213010098. However, new experiments seem to indicate its nonexistence: Zaitsevskii, Andréi; Schwarz, W H Eugen (tháng 4 năm 2014). “Structures and stability of AnO4 isomers, An = Pu, Am, and Cm: a relativistic density functional study”. Physical Chemistry Chemical Physics. 2014 (16): 8997–9001. doi:10.1039/c4cp00235k.
- ^ Es(IV) is known in einsteinium(IV) fluoride (EsF4); see Kleinschmidt, P (1994). “Thermochemistry of the actinides”. Journal of Alloys and Compounds. 213–214: 169–172. doi:10.1016/0925-8388(94)90898-2.
- ^ Db(V) has been observed in dubnium pentachloride (DbCl5); see H. W. Gäggeler (2007). “Gas Phase Chemistry of Superheavy Elements” (PDF). Paul Scherrer Institute. tr. 26–28. Bản gốc (PDF) lưu trữ ngày 20 tháng 2 năm 2012.
- ^ Sg(VI) has been observed in seaborgium oxide hydroxide (SgO2(OH)2); see Huebener; Taut, S.; Vahle, A.; Dressler, R.; Eichler, B.; Gäggeler, H. W.; Jost, D.T.; Piguet, D.; và đồng nghiệp (2001). “Physico-chemical characterization of seaborgium as oxide hydroxide” (PDF). Radiochim. Acta. 89 (11–12_2001): 737–741. doi:10.1524/ract.2001.89.11-12.737. Bản gốc (PDF) lưu trữ ngày 25 tháng 10 năm 2014.
- ^ Bh(VII) has been observed in bohrium oxychloride (BhO3Cl); see "Gas chemical investigation of bohrium (Bh, element 107)" Lưu trữ 2008-02-28 tại Wayback Machine, Eichler, GSI Annual Report 2000. Truy cập 2008-02-29
- ^ Hs(VIII) has been observed in hassium tetroxide (HsO4); see “Chemistry of Hassium” (PDF). Gesellschaft für Schwerionenforschung mbH. 2002. Truy cập ngày 31 tháng 1 năm 2007.
- ^ Cn(II) has been observed in copernicium selenide (CnSe); see Paul Scherrer Institute (2015). “Annual Report 2015: Laboratory of Radiochemistry and Environmental Chemistry” (PDF). Paul Scherrer Institute. tr. 3.Quản lý CS1: sử dụng tham số tác giả (liên kết)
- ^ Langmuir, Irving (1919). “The arrangement of electrons in atoms and molecules”. J. Am. Chem. Soc. 41 (6): 868–934. doi:10.1021/ja02227a002.