Thành viên:Naazulene/Beta oxy hóa
Beta oxy hóa hay oxy hóa acid béo là quá trình phân giải acid béo trong ti thể của sinh vật nhân thực hoặc trong bào tương của sinh vật nhân sơ để hình thành acetyl-CoA. Acetyl-CoA chính là "nguyên liệu đầu vào" của chu trình Krebs nhằm tạo ra "đồng xu năng lượng" ATP. Ngoài ra, beta oxy hóa còn tạo ra NADH và FADH2 để tham gia vào chuỗi chuyền điện tử.
Nó được đặt tên như vậy là vì sự oxy hóa diễn ra trên carbon beta của acid béo.
Quá trình này được thực hiện bởi ti thể và peroxisome, trong đó ti thể là bào quan chủ yếu, còn peroxisome chỉ chịu trách nhiệm cho những acid béo chuỗi rất dài.
Phương trình chung của beta oxy hóa:
Hoạt hóa và vận chuyển acid béo[sửa | sửa mã nguồn]
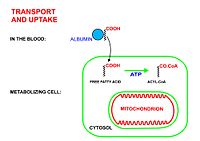
Acid béo tích điện âm nên không thể khuếch tán đơn giản qua màng phospholipid kép của ti thể. Vì vậy, nó cần sự hỗ trợ của một số enzyme thuộc họ SLC27 (họ protein vận chuyển acid béo) và ACSL (họ protein tổng hợp acyl-CoA).[1] Hai quá trình để nó thâm nhập vào ti thể là "hoạt hóa" và "vận chuyển".
Hoạt hóa[sửa | sửa mã nguồn]
Enzyme nối acid béo chuỗi dài—CoA (thuộc họ ACSL) xúc tác phản ứng giữa acid béo chuỗi dài với ATP để tạo tạo thành acid béo adenylate và pyrophosphate vô cơ, sau đó phản ứng với CoA để tạo thành acyl-CoA và AMP. Vì sản phẩm tạo thành là acyl-CoA, enzyme này đôi khi còn được gọi là acyl-CoA synthase.[2]
Vận chuyển[sửa | sửa mã nguồn]
Acyl-CoA có chuỗi dài (C12 - C22) được vận chuyển bằng thoi carnitine.
- Acyl-CoA được chuyển vào nhóm hydroxyl của carnitine bởi carnitine palmitoyltransferase I, một enzyme ở mặt tế bào chất của màng ti thể (cả màng trong và màng ngoài)
- Acyl-carnitine được chuyển vào trong ti thế bởi carnitine-acylcarnitine translocase
- Acyl-carnitine được chuyển trở lại thành acyl-CoA bởi carnitine palmitoyltransferase II, một enzyme ở mặt hướng vào chất nền của màng trong ti thể.
- Carnitine được giải phóng và sẽ được đối vận chuyển với acyl-carnitine để trở lại tế bào chất. Subsequently, a carnitine-acylcarnitine translocase transports Acyl-carnitine into the mitochondria, while simultaneously exporting carnitine out of the mitochondria.
Within the mitochondria, carnitine palmitoyltransferase II, positioned on the inner mitochondrial membrane's interior face, catalyzes the conversion of Acyl-carnitine back to acyl-CoA. The freed carnitine is then transported back to the cytosol, while an acyl-carnitine is conveyed into the mitochondrial matrix.
Acyl-CoA có chuỗi ngắn khuếch tán trực tiếp qua màng ti thể.
| Step-1 | Step-2 | Step-3 | Step-4 |
 |
 |
 |
 |
Cơ chế chung[sửa | sửa mã nguồn]

Sau khi acid béo được hoạt hóa vận chuyển vào chất nền ti thế, sự beta oxy hóa mới chính thức diễn ra. Acid béo (dưới dạng acyl-CoA) sẽ được cắt thành các acetyl-CoA cho đến hết mạch, mỗi lần cắt 2 carbon. Quá trình đó gồm bốn bước, mỗi bước tương ứng với một enzyme.[3]
- Dehydro hóa: acyl CoA dehydrogenase
- Hydrat hóa: enoyl-CoA hydratase
- Dehydro hóa: 3-hydroxyacyl CoA dehydrogenase
- Lưu phân: thiolase
Sản phẩm sau mỗi bốn bước này là một acetyl-CoA (nguyên liệu của chu trình Krebs), một NADH và FADH2 (nguyên liệu của chuỗi chuyền electron) và một acyl-CoA mới, ngắn hơn 2 carbon so với acyl-CoA ban đầu. Acyl-CoA mới tiếp tục tham gia beta oxy hóa cho đến hết chuỗi.
Nếu acyl-CoA ban đầu có số chẵn carbon (ví dụ như , nó sẽ phân hủy hết thành acetyl-CoA. Nếu acyl-CoA có số lẻ carbon, nó sẽ phân hủy đến khi còn 3 carbon.
Fatty acids are oxidized by most of the tissues in the body. However, some tissues such as the red blood cells of mammals (which do not contain mitochondria) and cells of the central nervous system do not use fatty acids for their energy requirements, but instead use carbohydrates (red blood cells and neurons) or ketone bodies (neurons only).
Because many fatty acids are not fully saturated or do not have an even number of carbons, several different mechanisms have evolved, described below.
Even-Numbered Saturated Fatty Acids[sửa | sửa mã nguồn]
Once inside the mitochondria, each cycle of β-oxidation, liberating a two carbon unit (acetyl-CoA), occurs in a sequence of four reactions[4]:
| Description | Diagram | Enzyme | End product |
| Dehydrogenation by FAD: The first step is the oxidation of the fatty acid by Acyl-CoA-Dehydrogenase. The enzyme catalyzes the formation of a trans-double bond between the C-2 and C-3 by selectively remove hydrogen atoms from the β-carbon. The regioselectivity of this step is essential for the subsequent hydration and oxidation reactions. |  |
acyl CoA dehydrogenase | trans-Δ2-enoyl-CoA |
| Hydration: The next step is the hydration of the bond between C-2 and C-3. The reaction is stereospecific, forming only the L isomer. Hydroxyl group is positioned suitable for the subsequent oxidation reaction by 3-hydroxyacyl-CoA dehydrogenase to create a β-keto group. |  |
enoyl CoA hydratase | L-β-hydroxyacyl CoA |
| Oxidation by NAD+: The third step is the oxidation of L-β-hydroxyacyl CoA by NAD+. This converts the hydroxyl group into a keto group. |  |
3-hydroxyacyl-CoA dehydrogenase | β-ketoacyl CoA |
| Thiolysis: The final step is the cleavage of β-ketoacyl CoA by the thiol group of another molecule of Coenzyme A. The thiol is inserted between C-2 and C-3. |  |
β-ketothiolase | An acetyl-CoA molecule, and an acyl-CoA molecule that is two carbons shorter |
This process continues until the entire chain is cleaved into acetyl CoA units. The final cycle produces two separate acetyl CoAs, instead of one acyl CoA and one acetyl CoA. For every cycle, the Acyl CoA unit is shortened by two carbon atoms. Concomitantly, one molecule of FADH2, NADH and acetyl CoA are formed.
Acid béo có số carbon lẻ[sửa | sửa mã nguồn]

Acid béo có số carbon lẻ (acid béo mạch lẻ) xuất hiện trong một số thực vật, hải sản và chế phẩm của động vật nhai lại (như sữa dê và mỡ bò).[5][6] Nó cũng bị beta oxy hóa y chang như acid béo mạch chẵn.
Sản phẩm cuối cùng của mỗi acid béo mạch lẻ là nhiều acetyl-CoA (2 carbon) và một propionyl-CoA (3 carbon). Propionyl-CoA tiếp tục bị oxy hóa để hình thành succinyl-CoA. Succinyl-CoA là một thành viên của chu trình Krebs.
Quá trình từ propionyl-CoA thành succinyl-CoA gồm các bước:
| Loại phản ứng | Chất phản ứng | Chất sản phẩm | Enzyme | Mô tả |
|---|---|---|---|---|
| Carboxyl hóa | propionyl-CoA | D-methylmalonyl CoA | propionyl-CoA carboxylase (coenzyme: biotin) | đặt một ion bicacbonat vào trong đồng phân lập thể dạng D của methylmalonyl-CoA, phản ứng này sẽ bao hàm sự tham gia của cofactor biotin, CO2 và ATP. Ion bicacbonat sẽ được cộng thêm vào cacbon giữa của propionyl-CoA, hình thành nên D-methylmalonyl-CoA. Sản phẩm là một chất 4C. |
| D-methylmalonyl CoA | L-methylmalonyl CoA | methylmalonyl-CoA epimerase | ||
| Đồng phân hóa | methylmalonyl CoA | succinyl-CoA | methylmalonyl-CoA mutase (coenzyme: vitamin B12) | thay đổi cấu trúc của L-methylmalonyl-CoA (yêu cầu sự tham gia của B12 với tư cách là coenzyme) để hình thành succinyl-CoA, một thành viên của chu trình Krebs. |
Tuy nhiên, trong khi acetyl-CoA phải ngưng tụ với oxaloacetate mới có thể tham gia chu trình Krebs, succinyl-CoA có thể cứ thế mà xấn vào chu trình vì bản thân nó đã là một sản phẩm chuyển hóa của chu trình Krebs. Vì vậy, succinyl-CoA có thể bị dư thừa và "chuyển công tác" sang con đường tân tạo đường.[7]
Acid béo không bão hòa[sửa | sửa mã nguồn]
Vấn đề của acid béo không bão hòa là liên kết đôi cis của nó có thể ngăn cản sự hình thành liên kết trans-Δ2. Hai enzyme đi giải quyết vấn đề này là enoyl-CoA isomerase và 2,4 dienoyl CoA reductase.[8]
Acid béo không bão hòa tham gia beta oxy hóa cho đến khi mắc kẹt ở một liên kết đôi cis. Lúc đó hai enzyme kia ra tay:
- Liên kết đôi vị trí chẵn sẽ được enzyme isomerase chuyển từ dạng cis thành dạng trans.
- Liên kết đôi vị trí lẻ sẽ sẽ được enzyme reductase chuyển sang vị trí chẵn để isomerase có thể xử lí

β-oxidation occurs normally until the acyl CoA (because of the presence of a double bond) is not an appropriate substrate for acyl CoA dehydrogenase, or enoyl CoA hydratase:
- If the acyl CoA contains a cis-Δ3 bond, then cis-Δ3-Enoyl CoA isomerase will convert the bond to a trans-Δ2 bond, which is a regular substrate.
- If the acyl CoA contains a cis-Δ4 double bond, then its dehydrogenation yields a 2,4-dienoyl intermediate, which is not a substrate for enoyl CoA hydratase. However, the enzyme 2,4 Dienoyl CoA reductase reduces the intermediate, using NADPH, into trans-Δ3-enoyl CoA. This compound is converted into a suitable intermediate by 3,2-Enoyl CoA isomerase and β-Oxidation continues.
Peroxisomal Beta-Oxidation[sửa | sửa mã nguồn]
Fatty acid oxidation also occurs in peroxisomes when the fatty acid chains are too long to be processed by the mitochondria. The same enzymes are used in peroxisomes as in the mitochondrial matrix and acetyl-CoA is generated. Very long chain (greater than C-22) fatty acids, branched fatty acids,[9] some prostaglandins and leukotrienes[10] undergo initial oxidation in peroxisomes until octanoyl-CoA is formed, at which point it undergoes mitochondrial oxidation.[11]
One significant difference is that oxidation in peroxisomes is not coupled to ATP synthesis. Instead, the high-potential electrons are transferred to O2, which yields hydrogen peroxide. The enzyme catalase, found primarily in peroxisomes and the cytosol of erythrocytes (and sometimes in mitochondria[12]), converts the hydrogen peroxide into water and oxygen.
Peroxisomal β-oxidation also requires enzymes specific to the peroxisome and to very long fatty acids. There are four key differences between the enzymes used for mitochondrial and peroxisomal β-oxidation:
- The NADH formed in the third oxidative step cannot be reoxidized in the peroxisome, so reducing equivalents are exported to the cytosol.
- β-oxidation in the peroxisome requires the use of a peroxisomal carnitine acyltransferase (instead of carnitine acyltransferase I and II used by the mitochondria) for transport of the activated acyl group into the mitochondria for further breakdown.
- The first oxidation step in the peroxisome is catalyzed by the enzyme acyl-CoA oxidase.
- The β-ketothiolase used in peroxisomal β-oxidation has an altered substrate specificity, different from the mitochondrial β-ketothiolase.
Peroxisomal oxidation is induced by a high-fat diet and administration of hypolipidemic drugs like clofibrate.
Energy yield[sửa | sửa mã nguồn]
Even-numbered saturated fatty acids
Theoretically, the ATP yield for each oxidation cycle where two carbons are broken down at a time is 17, as each NADH produces 3 ATP, FADH2 produces 2 ATP and a full rotation of Acetyl-CoA in citric acid cycle produces 12 ATP.[13] In practice, it is closer to 14 ATP for a full oxidation cycle as 2.5 ATP per NADH molecule is produced, 1.5 ATP per each FADH2 molecule is produced and Acetyl-CoA produces 10 ATP per rotation of the citric acid cycle [13](according to the P/O ratio). This breakdown is as follows:
| Source | ATP | Total |
| 1 FADH2 | x 1.5 ATP | = 1.5 ATP (Theoretically 2 ATP)[13] |
| 1 NADH | x 2.5 ATP | = 2.5 ATP (Theoretically 3 ATP)[13] |
| 1 Acetyl CoA | x 10 ATP | = 10 ATP (Theoretically 12 ATP) |
| 1 Succinyl CoA | x 4 ATP | = 4 ATP |
| Total | = 14 ATP |
For an even-numbered saturated fat (Cn), 0.5 * n - 1 oxidations are necessary, and the final process yields an additional acetyl CoA. In addition, two equivalents of ATP are lost during the activation of the fatty acid. Therefore, the total ATP yield can be stated as:
or
For instance, the ATP yield of palmitate (C16, n = 16) is:
Represented in table form:
| Source | ATP | Total |
| 7 FADH2 | x 1.5 ATP | = 10.5 ATP |
| 7 NADH | x 2.5 ATP | = 17.5 ATP |
| 8 Acetyl CoA | x 10 ATP | = 80 ATP |
| Activation | = -2 ATP | |
| Total | = 106 ATP |
Odd-numbered saturated fatty acid

For an odd-numbered saturated fat (Cn), 0.5 * n - 1.5 oxidations are necessary, and the final process yields 8 acetyl CoA and 1 propionyl CoA. It is then converted to a succinyl CoA by a carboxylation reaction and generates additional 5 ATP (1 ATP is consumed in carboxylation process generating a net of 4 ATP). In addition, two equivalents of ATP are lost during the activation of the fatty acid. Therefore, the total ATP yield can be stated as:
or
For instance, the ATP yield of Nonadecylic acid (C19, n = 19) is:
Represented in table form:
| Source | ATP | Total |
| 8 FADH2 | x 1.5 ATP | = 12 ATP |
| 8 NADH | x 2.5 ATP | = 20 ATP |
| 8 Acetyl CoA | x 10 ATP | = 80 ATP |
| 1 Succinyl CoA | x 4 ATP | = 4 ATP |
| Activation | = -2 ATP | |
| Total | = 114 ATP |
Clinical significance[sửa | sửa mã nguồn]
There are at least 25 enzymes and specific transport proteins in the β-oxidation pathway.[16] Of these, 18 have been associated with human disease as inborn errors of metabolism.
Furthermore, studies indicate that lipid disorders are involved in diverse aspects of tumorigenesis, and fatty acid metabolism makes malignant cells more resistant to a hypoxic environment. Accordingly, cancer cells can display irregular lipid metabolism with regard to both fatty acid synthesis and mitochondrial fatty acid oxidation (FAO) that are involved in diverse aspects of tumorigenesis and cell growth.[17]
Some β-oxidation disorders are-
1. Medium-chain acyl-coenzyme A dehydrogenase (MCAD) Deficiency[18]
It is the most common fatty acid β-oxidation disorder and a prevalent metabolic congenital error It is often identified through newborn screening. Although children are normal at birth, symptoms usually emerge between three months and two years of age, with some cases appearing in adulthood.
Medium-chain acyl-CoA dehydrogenase (MCAD) plays a crucial role in mitochondrial fatty acid β-oxidation, a process vital for generating energy during extended fasting or high-energy demand periods. This process, especially important when liver glycogen is depleted, supports hepatic ketogenesis. The specific step catalyzed by MCAD involves the dehydrogenation of acyl-CoA. This step converts medium-chain acyl-CoA to trans-2-enoyl-CoA, which is then further metabolized to produce energy in the form of ATP.
Symptoms
- Affected children, who seem healthy initially, may experience symptoms like low blood sugar without ketones (hypoketotic hypoglycemia) and vomiting
- Can escalate to lethargy, seizures and coma, typically triggered by illness
- Acute episodes may also involve enlarged liver (hepatomegaly) and liver issues
- Sudden death
Treatments
- Administering simple carbohydrates
- Avoiding fasting
- Frequent feedings for infants
- For toddlers, a diet with less than 30% of total energy from fat
- Administering 2 g/kg of uncooked cornstarch at bedtime for sufficient overnight glucose
- Preventing hypoglycemia, especially due to excessive fasting.
- Avoiding infant formulas with medium-chain triglycerides as the main fat source

2. Long-Chain Hydroxyacyl-CoA Dehydrogenase (LCHAD) Deficiency [19]
LCHAD performs the dehydrogenation of hydroxyacyl-CoA derivatives, facilitating the removal of hydrogen and the formation of a keto group. This reaction is essential for the subsequent steps in beta oxidation that lead to the production of acetyl-CoA, NADH, and FADH2, which are important for generating ATP, the energy currency of the cell.
Long-chain hydroxyacyl-CoA dehydrogenase (LCHAD) deficiency is a condition that affects mitochondrial function due to enzyme impairments. LCHAD deficiency is specifically caused by a shortfall in the enzyme long-chain 3-hydroxyacyl-CoA dehydrogenase. This leads to the body's inability to transform specific fats into energy, especially during fasting periods.
Symptoms
- Severe Phenotype: symptoms appear soon after birth and include hypoglycemia, hepatomegaly, brain dysfunction (encephalopathy) and often cardiomyopathy
- Intermediate Phenotype: characterized by hypoketotic hypoglycemia and is triggered by infection or fasting during infancy
- Mild (Late-Onset) Phenotype: presents as muscle weakness (myopathy) and nerve disease (neuropathy)
- Long-Term Complications: can include peripheral neuropathy and eye damage (retinopathy)
Treatments
- Regular feeding to avoid fasting
- Use of medium-chain triglyceride (MCT) or triheptanoin supplements and carnitine supplements
- Low-fat diet
- Hospitalization with intravenous fluids containing at least 10% dextrose
- Bicarbonate therapy for severe metabolic acidosis
- Management of high ammonia levels and muscle breakdown
- Cardiomyopathy management
- Regular monitoring of nutrition, blood and liver tests with annual fatty acid profile
- Growth, development, heart and neurological assessments and eye evaluations
3. Very Long-Chain Acyl-Coenzyme A Dehydrogenase (VLCAD) Deficiency
In the β-oxidation cycle, VLCAD's role involves the removal of two hydrogen atoms from the acyl-CoA molecule, forming a double bond and converting it into trans-2-enoyl-CoA. This step is essential for the fatty acid to undergo further processing and energy production.
Very Long-Chain Acyl-Coenzyme A Dehydrogenase Deficiency is a genetic disorder that affects the body's ability to break down certain fats. It is caused by a deficiency in the enzyme very long-chain acyl-coenzyme A dehydrogenase. This enzyme is crucial for the first step of mitochondrial beta-oxidation. Without this enzyme, the body struggles to effectively break down long-chain fatty acids. This can lead to a buildup of these fats and a shortage of energy, particularly during periods of fasting or increased physical activity.[20]
Symptoms
- Severe Early-Onset Cardiac and Multiorgan Failure Form: symptoms appear within days of birth and include hypertrophic/dilated cardiomyopathy, fluid around heart (pericardial effusion), heart rhythm problems (arrhythmias), hepatomegaly and occasional intermittent hypoglycemia
- Hepatic or Hypoketotic Hypoglycemic Form: typically appears in early childhood with hypoketotic hypoglycemia
- Later-Onset Episodic Myopathic Form: presents with muscle breakdown after exercise (intermittent rhabdomyolysis), muscle cramps and pain, exercise intolerance and low blood sugar
Treatments
- Low-fat diet
- Use of medium-chain triglyceride (MCT) supplements
- Regular, frequent feeding, especially for infants and children
- Snacks high in complex carbohydrates before bedtime
- Guided and limited exercise for older individuals
- Administration of high-energy fluids intravenously
- Avoiding L-carnitine and IV fats
- Plenty of fluids and urine alkalization for muscle breakdown
See also[sửa | sửa mã nguồn]
- Fatty acid metabolism
- Fatty-acid metabolism disorder
- Lipolysis
- Krebs cycle
- Omega oxidation
- Alpha oxidation
References[sửa | sửa mã nguồn]
- ^ Anderson, Courtney M.; Stahl, Andreas (2013). “SLC27 fatty acid transport proteins”. Molecular Aspects of Medicine (bằng tiếng Anh). 34 (2–3): 516–528. doi:10.1016/j.mam.2012.07.010.
- ^ “ExplorEnz: EC 6.2.1.3”. www.enzyme-database.org. Truy cập ngày 6 tháng 12 năm 2023.
- ^ Houten, Sander Michel; Wanders, Ronald J. A. (2010). “A general introduction to the biochemistry of mitochondrial fatty acid β‐oxidation”. Journal of Inherited Metabolic Disease (bằng tiếng Anh). 33 (5): 469–477. doi:10.1007/s10545-010-9061-2. ISSN 0141-8955.
- ^ Talley, Jacob T.; Mohiuddin, Shamim S. (2023), “Biochemistry, Fatty Acid Oxidation”, StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 32310462, truy cập ngày 3 tháng 12 năm 2023
- ^ Nelson DL, Cox MM (2005). Lehninger Principles of Biochemistry (ấn bản 4). New York: W. H. Freeman and Company. tr. 648–649. ISBN 978-0-7167-4339-2.
- ^ Rodwell VW. Harper's Illustrated Biochemistry (ấn bản 31). McGraw-Hill Publishing Company.
- ^ King M. “Gluconeogenesis: Synthesis of New Glucose”. Subsection: "Propionate". themedicalbiochemistrypage.org, LLC. Truy cập ngày 20 tháng 3 năm 2013.
- ^ Schulz, Horst (28 tháng 1 năm 1991). “Beta oxidation of fatty acids”. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 1081 (2): 109–120. doi:10.1016/0005-2760(91)90015-A. ISSN 0005-2760.
- ^ Singh I (tháng 2 năm 1997). “Biochemistry of peroxisomes in health and disease”. Molecular and Cellular Biochemistry. 167 (1–2): 1–29. doi:10.1023/A:1006883229684. PMID 9059978. S2CID 22864478.
- ^ Gibson GG, Lake BG (8 tháng 4 năm 2013). Peroxisomes: Biology and Importance in Toxicology and Medicine. CRC Press. tr. 69–. ISBN 978-0-203-48151-6.
- ^ Lazarow PB (tháng 3 năm 1978). “Rat liver peroxisomes catalyze the beta oxidation of fatty acids”. The Journal of Biological Chemistry. 253 (5): 1522–8. doi:10.1016/S0021-9258(17)34897-4. PMID 627552.
- ^ Bai J, Cederbaum AI (2001). “Mitochondrial catalase and oxidative injury”. Biological Signals and Receptors. 10 (3–4): 3189–199. doi:10.1159/000046887. PMID 11351128. S2CID 33795198.
- ^ a b c d Rodwell, Victor (2015). Harper's illustrated Biochemistry, 30th edition. USA: McGraw Hill Education. tr. 164. ISBN 978-0-07-182537-5.
- ^ Jain P, Singh S, Arya A (tháng 1 năm 2021). “A student centric method for calculation of fatty acid energetics: Integrated formula and web tool”. Biochemistry and Molecular Biology Education. 1 (1): 492–499. doi:10.1002/bmb.21486. PMID 33427394. S2CID 231577993.
- ^ “Biosynthesis of Iso-Fatty Acids in Myxobacteria: Iso-Even Fatty Acids Are Derived by a-Oxidation from Iso-Odd Fatty Acids”. Truy cập ngày 7 tháng 11 năm 2023.
- ^ Tein I (2013). “Disorders of fatty acid oxidation”. Pediatric Neurology Part III. Handbook of Clinical Neurology. 113. tr. 1675–88. doi:10.1016/B978-0-444-59565-2.00035-6. ISBN 9780444595652. PMID 23622388.
- ^ Ezzeddini R, Taghikhani M, Salek Farrokhi A, Somi MH, Samadi N, Esfahani A, Rasaee, MJ (tháng 5 năm 2021). “Downregulation of fatty acid oxidation by involvement of HIF-1α and PPARγ in human gastric adenocarcinoma and its related clinical significance”. Journal of Physiology and Biochemistry. 77 (2): 249–260. doi:10.1007/s13105-021-00791-3. PMID 33730333. S2CID 232300877.
- ^ Vishwanath, Vijay A. (2016). “Fatty Acid Beta-Oxidation Disorders: A Brief Review”. Annals of Neurosciences (bằng tiếng Anh). 23 (1): 51–55. doi:10.1159/000443556. ISSN 0972-7531.
- ^ Prasun, Pankaj; LoPiccolo, Mary Kate; Ginevic, Ilona (1993), Adam, Margaret P.; Feldman, Jerry; Mirzaa, Ghayda M.; Pagon, Roberta A. (eds.), "Long-Chain Hydroxyacyl-CoA Dehydrogenase Deficiency / Trifunctional Protein Deficiency", GeneReviews®, Seattle (WA): University of Washington, Seattle, PMID 36063482, retrieved 2023-12-03
- ^ Leslie, Nancy D.; Saenz-Ayala, Sofia (1993), Adam, Margaret P.; Feldman, Jerry; Mirzaa, Ghayda M.; Pagon, Roberta A. (biên tập), “Very Long-Chain Acyl-Coenzyme A Dehydrogenase Deficiency”, GeneReviews®, Seattle (WA): University of Washington, Seattle, PMID 20301763, truy cập ngày 4 tháng 12 năm 2023
Further reading[sửa | sửa mã nguồn]
- Berg JM, Tymoczko JL, Stryer L (2002). “Certain Fatty Acids Require Additional Steps for Degradation”. Biochemistry (ấn bản 5). New York: W H Freeman. ISBN 978-0-7167-4684-3.
External links[sửa | sửa mã nguồn]
- Silva P. “The chemical logic behind fatty acid metabolism”. Universidade Fernando Pessoa. Bản gốc lưu trữ ngày 16 tháng 3 năm 2010.
- “Fatty acid oxidation animation”. Cengage Learning. Bản gốc lưu trữ ngày 8 tháng 5 năm 2012. Truy cập ngày 2 tháng 5 năm 2007.
- Jain, P.; Singh, S.; Arya, A. (2021). “Integrated formulae for calculating fatty acid ATP yield”. Biochemistry and Molecular Biology Education. 49 (3): 492–499. doi:10.1002/bmb.21486. PMID 33427394. S2CID 231577993.
Bản mẫu:Lipid metabolism enzymes Bản mẫu:Fatty-acid metabolism disorders